
How does LUXTURNA work?
How LUXTURNA® works
A one-time gene therapy for each eye
Gene therapy is a method for treating or preventing genetic disease. One approach to gene therapy is delivering a new or functional gene into a cell, which is how LUXTURNA works.
Working to restore the visual cycle
The visual cycle is a process that allows you to see. RPE65 is a key gene in the visual cycle. When not working properly, vision deteriorates and may result in blindness. LUXTURNA provides a working RPE65 gene to act in place of a mutated RPE65 gene. This working gene has the potential to make the visual cycle work properly again.
-
 With the gene delivered by LUXTURNA, cells in the retina of the eye can produce the RPE65 protein.
With the gene delivered by LUXTURNA, cells in the retina of the eye can produce the RPE65 protein.
-
 The RPE65 protein makes the visual cycle work properly.
The RPE65 protein makes the visual cycle work properly.
-
 Once the visual cycle is working properly, vision can be improved.
Once the visual cycle is working properly, vision can be improved.
Clinical trial results
How the effect of LUXTURNA was measured
You may be used to tests such as visual acuity, visual field, and light sensitivity. These tests measure visual function, which is how vision loss affects your eyes.

A new test was created to measure functional vision, which is how vision loss affects the ability to perform daily life activities. This test is called the Multi-Luminance Mobility Test® (MLMT®).
In the MLMT, participants had to navigate a course of obstacles under different light levels. The light levels went from bright to dark.
The clinical trial included 31 participants, four to 44 years old. Participants were eligible for the clinical trial if they:
- Were three years of age or older
- Had a genetic diagnosis of mutations in both copies of the RPE65 gene
- Had enough remaining cells in their retina as determined by a healthcare professional
- Had visual acuity of 20/60 or worse in both eyes and/or visual field less than 20 degrees
- Were able to perform the Multi-Luminance Mobility Test® within the light level range evaluated, but unable to pass at the lowest light level
Two groups of participants were treated with LUXTURNA®, each at a different time. The first group was treated before the second group in order to compare results between treated and untreated participants. The second group was then treated one year after the first group.
- LUXTURNA was administered to the participants’ first eye and, six to 18 days later, LUXTURNA was administered to their second eye
- Participants followed a prescribed course of medication to reduce risks related to an immune response
- Each participant was evaluated 1 year after treatment to measure the effect of LUXTURNA
LUXTURNA has more than 12 years of safety experience and has been studied in a range of patients aged between four and 44 years
In clinical trials, LUXTURNA improved functional vision. This increased participants’ ability to perform daily life activities
Two groups of participants were treated with LUXTURNA, each at a different time. The first group was treated before the second group in order to compare results between treated and untreated participants. The second group was then treated one year after the first group.
One year after treatment, participants in the first group were able to navigate through the MLMT course using both eyes at two light levels darker than before treatment.

of participants in both groups (16 of 29) showed an improvement of at least two light levels darker after treatment
A majority of participants were able to complete the course at the lowest light level after treatment
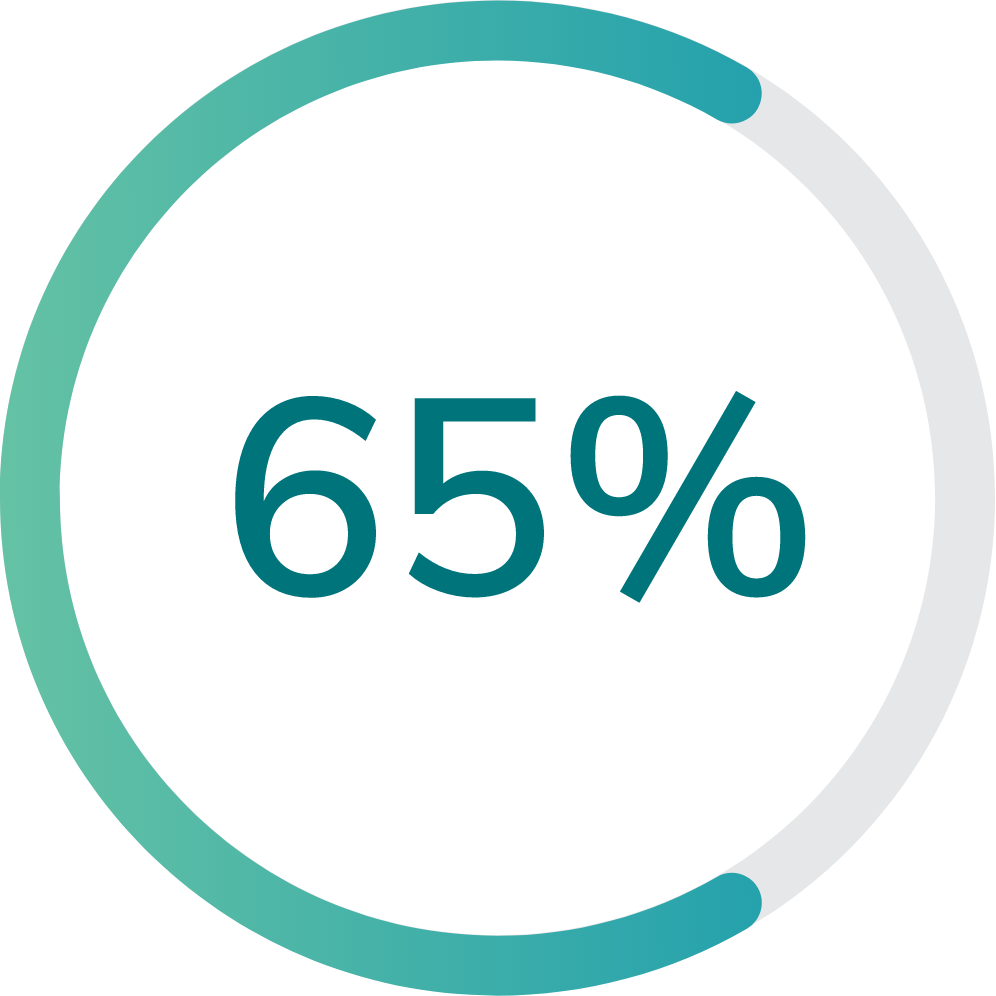
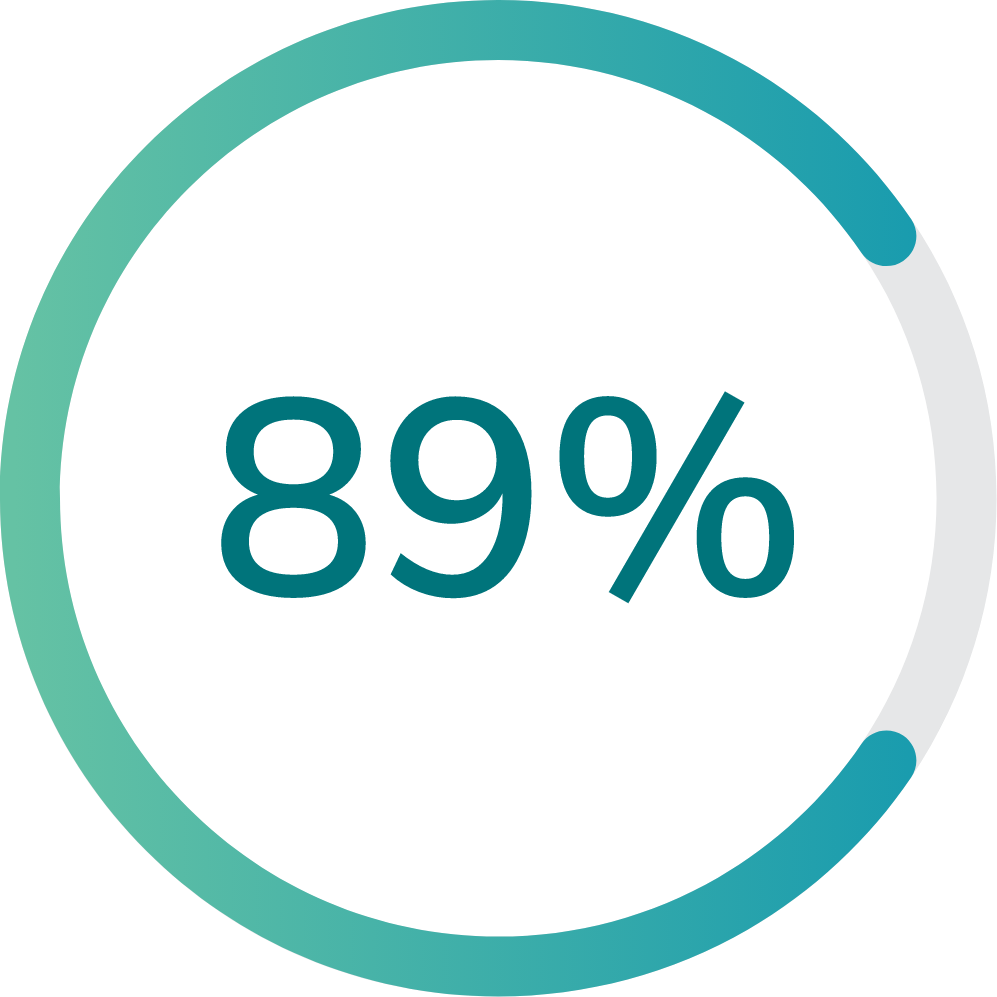
65% (13 of 20) of participants in the first group and 89% (8 of 9) of participants in the second group were able to navigate through the Multi-Luminance Mobility Test (MLMT) course at the lowest light level, which was equal to a moonless summer night.
First group
Second group
Watch the videos below to see how LUXTURNA improved functional vision in the clinical trials and maintained it across five years
The Multi-Luminance Mobility Test® (MLMT®) in action
See the difference in patients’ ability to navigate through the MLMT course before treatment and one year after treatment with LUXTURNA.
Before treatment at the lowest light level (fail)
One year after treatment at the lowest light level (pass)
This video shows a LUXTURNA clinical trial participant navigating the Multi-Luminance Mobility Test course unsuccessfully at the lowest light level before treatment. One year after treatment with LUXTURNA, the participant tries again and successfully passes the course at the lowest light level.
The camera used automatically adjusts the level and temperature of light that it captures. Because of this feature, there may be slight variations in shade when filming at low light levels. Both videos were filmed in low-light environments.

These improvements in functional vision were maintained across
Observations will continue for up to 15 years after treatment.
A LUXTURNA clinical trial participant navigating the MLMT course at the lowest light level five years after treatment.
This video shows a LUXTURNA clinical trial participant navigating and successfully passing the MLMT course five years after treatment.
How LUXTURNA is administered
LUXTURNA is given as a surgical injection beneath the retina of each eye by a healthcare professional knowledgeable in the administration of the therapy
One eye is treated at a time. After the first eye is treated, the second eye will be treated at least six days later.
Administration is performed at specific ocular gene therapy treatment centers
Each treatment center is staffed with:
- Healthcare professionals, including retina specialists
- Nurses
- Genetic counselors
The staff at the treatment centers has experience treating patients with IRDs and the retinal surgeons have been specifically educated on administration of LUXTURNA
Find treatment centerSide effect profile
The following serious side effects may occur during or after the administration of LUXTURNA:
- Eye infections, including a serious infection inside of the eye called endophthalmitis, that may lead to blindness.
- Permanent decline in visual acuity, or the sharpness of central vision.
- Changes in the retina (the thin layer of tissue in the back of the eye) that can lead to vision loss including:
- development of a hole, thinning, or loss of function of the retina, separation of the layers in the center of the retina, decreased thickness of the retina and the choroid (the layer of blood vessels that lines the back of the eye), or bleeding in the retina.
- breaks in or wrinkling on the surface of the retina or detachment of the retina.
- Increased pressure inside of the eye. You should follow-up with your healthcare professional as instructed to detect and treat any increased pressure in the eye as this may cause blindness.
- Expansion of the air bubble formed in the eye after administration of LUXTURNA. You should avoid air travel, travel to high elevations, or scuba diving until your healthcare professional has told you that the air bubble formed in the eye following administration of LUXTURNA has disappeared. Engaging in these activities while the air bubble is present can cause permanent vision loss.
- Formation or worsening of cataract (clouding of the lens inside of the eye).
The most common side effects that may occur with LUXTURNA are:
- Redness of the eye
- Cataract (clouding of the lens inside the eye)
- Increased pressure inside of the eye
- Breaks in the retina
- Dellen (thinning of the clear layer in the front of the eye)
- Development of a hole in the center of the retina
- Subretinal deposits (deposits under the retina)
- Eye swelling, irritation, or pain
- Wrinkling on the surface of the center of the retina
You should discuss any side effects with your healthcare professional.
Please see additional Important Safety Information for LUXTURNA.